We know that two like or similar charges repel each other and two opposite charges attract each other ( To know more about electric charge click here... ). In 1785 French physicist Charles Augustin de Coulomb determined the magnitude of this attraction and repulsion force and published a law about it. This law is known as Coulomb's Law. Here we will discuss this law
What is Coulomb's Law?
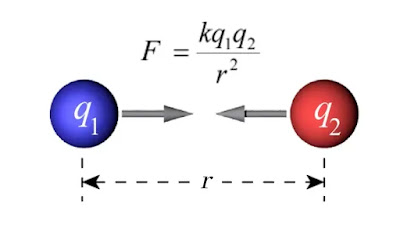
According to Coulomb’s law, the effective force of attraction or repulsion between two stationary point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
This force acts along the connecting line of the two charges and its value depends on the nature of the medium in which the charges are located.
Coulomb's formula: Consider, two points charges q1 and q2, are at a distance of r from each other. If the magnitude of the force is F, then according to Coulomb's law
Here k is a constant known as the electrostatic force constant or coulomb constant. It depends on the units of F, r, q1, q2, and on the nature of the medium in which the charges are located.
Coulomb's law in SI unit system
If the two charges are in a vacuum, then in the SI unit system k = 1/4𝜋𝜀o. Where 𝜀o is the permittivity of free-space/vacuum.
Now if the two charges are in the real medium, then k = 1/4𝜋𝜀. Where 𝜀 is the permittivity of that medium.
So in the SI unit system the Coulomb's law written as
Coulomb's law in CGS unit system
In the CGS unit system k = 1/K, Where K is the dielectric constant of the medium. So in the CGS unit system the Coulomb's law written as
If the medium is taken as a vacuum then K = 1, so the law can be written as
Read next: What is the Permittivity of Free Space?

Post a Comment (0)