What is Electric Charge?
In physics, charge, also known as electric charge, is the basic property of matter that gives rise to all electrical and magnetic forces and interactions. An electric charge is associated with a field called an electric field, but when it moves it generates a magnetic field around it. The combination of these electric and magnetic fields is also known as the electromagnetic field.
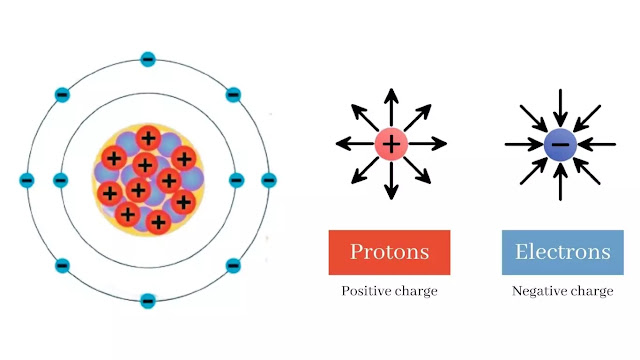
There are two types of electric charges: positive and negative electric charges. In atoms, the electron carries the negative charge and the proton carries the positive charge. In the atom, an electric charge occurs whenever the number of electrons surrounding the nucleus differs from the number of protons present in the nucleus.
When the number of electrons is more than the number of protons the atom gets a negative charge. But If there is more proton than the electron then the atom gets a positive charge.
For example, sodium cation (Na+) has 11 protons in the nucleus and 10 electrons around the nucleus so it is a positive charge, whereas chloride anion (Cl-) has 17 protons in the nucleus and 18 electrons around the nucleus so it is a negative charge.
Properties of Electric Charges?
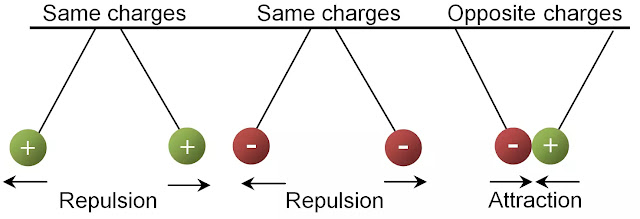
Two important properties of electric charges: 1. Two like or similar charges repel each other and 2. Two opposite charges attract each other.
Principle of Conservation of Charge
Principle of Conservation of Charge: Conservation of charge states that the total charge of an isolated system is always constant i.e it does not change with time.
According to this principle, charges can neither be created nor be destroyed. It is only possible to transfer charges from one part of an isolated system to another.
What is Quantisation of Charge?
All the charges found in nature are found to be integral multiples of the fundamental charge which is the charge of an electron. The charge of electrons can always be represented in the form of e, where the value of e is about 1.602×10 -19 C.
The value of this charge can no longer be divided, only the integral multiple of this charge is possible. This is referred to as quantization of charge.
So, the quantization of charge implies that any charged particle can possess a charge equivalent to some integral multiples of e, i.e.,
Q = ne, where n = 1, 2, 3, …
Thus, the charge cannot take any arbitrary values but only values that are integral multiples of the fundamental charge.
Continue Reading...

Post a Comment (0)