What Is Electromotive Force (EMF)?
Definition: Electromotive force (EMF) is an electric potential or a voltage produced by any source of electrical energy such as an electric generator or a battery.
An electric cell or battery is a device in which a constant difference in potential is maintained between the two conductors (called electrodes or terminals) by a chemical reaction. Thus a cell is used as a source of current that continuously provides energy to a circuit.
For example, a force must be applied from outside to maintain the flow of water in a pipe. Similarly, a conductor needs something like a force to keep the current flowing. This physical quantity, similar to the force supplied by an electric source, is known as electromotive force.
But force and electromotive force are two different physical quantities. Here the term force is somehow misleading because the electromotive force is not actually a force, it is the potential that provides energy to the circuit.
The name EMF is retained because of its historical reasons and it is useful to distinguish between the energy that is generated from the source and energy that is lost to resistors.
When a charged particle is present in a source, the EMF supplies the particle with kinetic energy which is what drives electric circuits. This energy can then be lost as heat throughout the circuit as the moving current of charges meets electrical resistance.
This resistance dictates how much current will flow into the circuit and can be measured by Ohm's law (V = IR). Here V is the voltage across the resistance.
Electromotive Force (EMF) of a Cell
When a cell is connected in a circuit containing various electrical components, the emf between the terminals causes the charge to flow through the components joined in the circuit.
So the emf of a cell is the energy spent (or the work done) per unit charge in taking a positive charge around the complete circuit of the cell (i.e in the circuit outside the cell and in the electrolyte inside the cell).
If W work is done in taking a test charge q around the complete circuit of the cell, then e.m.f. of the cell is
Now let's know about the unit and dimension of the electromotive force.
Unit & Dimension of EMF?
SI unit for Electromotive Force: The SI unit for electromotive force is Joules/Coulomb or Volt (V).
Numerically EMF is expressed as the number of Joules of energy given by the source divided by each Coulomb of charge to enable a unit electric charge to move across the circuit.
Dimension of Electromotive Force: As we already know EMF is the ratio of work done on a unit charge, so
Therefore, the dimension of the EMF is M¹L²T⁻³I⁻¹.
Factors affecting the emf of a cell
The emf of a cell is the characteristic that is different for different kinds of cells. For example, the emf of a voltaic cell is 1.08 volts, for a Leclanche cell it is 1.5 volts and for Danielcell it is 1.08 volts.
The emf of a cell depends on the following two factors:
(i) the material of the electrodes, and
(ii) the electrolyte used in the cell.
Note: The emf of a cell does not depend on the shape of electrodes, the distance between the electrodes, and the amount of electrolyte in it. And also the emf of a cell does not depend on the amount of current drawn from the cell.
EMF and Terminal Voltage
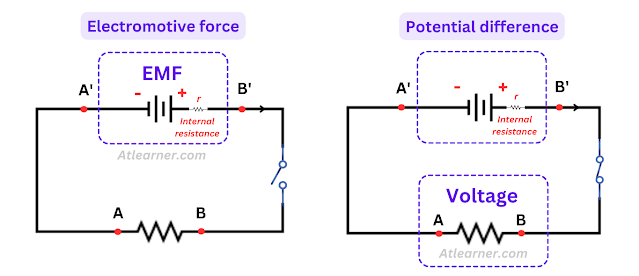
Electromotive Force: When no current is drawn from the source (cell or battery), the voltage between the electrodes of the source is called its electromotive force.
It is an open-circuit voltage and independent of the resistance of the electrical circuit. But it is dependent on the internal resistance of the circuit.
Actually, internal resistance is the resistance offered by electrolytes inside the cell. For this internal resistance, some amount of voltage drops happen.
If we consider I as the current drawn from the cell and r as internal resistance then the voltage drop v = Ir. Now if V is the terminal voltage then the emf of the cell is
Where ε is the emf and R is the resistance of the circuit.
Terminal voltage: When current is drawn from the source (cell or battery), the voltage between the electrodes of the source is called it's terminal voltage.
It is a closed-circuit voltage and is dependent on the resistance of the electrical circuit. If I is the current flow through the circuit and R is the resistance of the circuit then terminal voltage V = IR.
Therefore the formula for the terminal voltage with emf is
When a larger current is drawn from the cell, a greater number of charge carriers flow through the electrolyte.
Hence more work is done for moving the charges from one electrode to the other through the electrolyte, so more is the voltage drop v and therefore less is the terminal voltage.
Thus, the terminal voltage of a cell depends on the amount of current drawn from the cell.
Difference between EMF and Voltage
There is a difference between EMF and voltage -
One of the major differences is voltage causes current to flow between two points where EMF is the energy supplied to the charge.
EMF maintains the potential difference or voltage between two electrodes i.e EMF has constant intensity with a higher magnitude where the intensity of voltage is lower than EMF and it is non-constant.
The EMF on any source is measured by using a potentiometer where the voltage in an electric circuit is measured by a voltmeter.
Continue Reading...

Post a Comment (0)